Exploring the Chemical Reactions of hcooch ch2 h2o: Implications and Applications
The compound HCOOCH₂H₂O, commonly known as methyl formate hydrate, is an organic ester formed by the reaction of formic acid (HCOOH) with methanol (CH₃OH), followed by hydration. This compound is significant in various chemical processes and applications, particularly in organic synthesis, environmental chemistry, and industrial manufacturing.
Understanding HCOOCH₂H₂O: Structure and Composition
Chemical Structure
Methyl formate hydrate consists of a formate ester group (HCOO), a methylene group (CH₂), and a water molecule (H₂O). The chemical structure can be represented as:
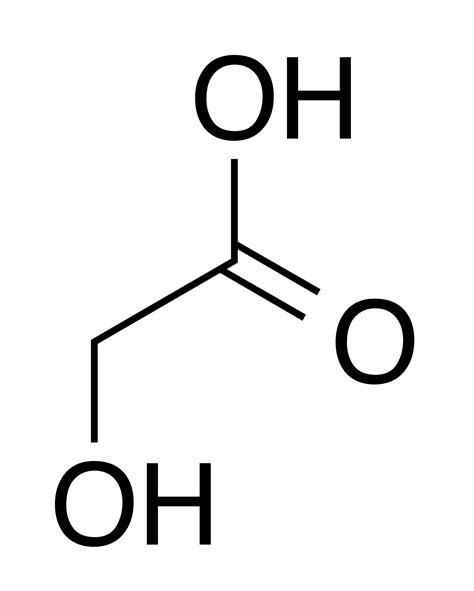
HCOO-CH₂-OH·H₂O
In this structure:
- HCOO: Formate ester group derived from formic acid.
- CH₂OH: Hydroxymethyl group originating from methanol.
- H₂O: Water molecule associated with the ester.
This combination imparts unique chemical properties to the compound, making it versatile in various reactions.
Physical Properties
Methyl formate hydrate is a colorless liquid with a faint, pleasant odor. It is highly soluble in water and exhibits moderate volatility. The presence of the water molecule in its structure influences its boiling point and reactivity.
Chemical Reactions Involving HCOOCH₂H₂O
Ester Hydrolysis
One of the primary reactions involving HCOOCH₂H₂O is ester hydrolysis. Under acidic or basic conditions, the ester bond in methyl formate hydrate can be broken down by water, leading to the formation of formic acid and methanol:
HCOOCH₂OH + H₂O → HCOOH + CH₃OH
This reaction is significant in laboratory and industrial settings, particularly in producing formic acid and methanol.
Transesterification
Methyl formate hydrate can undergo transesterification reactions; another alcohol replaces the methanol group. This process is crucial in biodiesel production, where methyl formate hydrate reacts with fatty acids to produce fatty acid esters and methanol.
Esterification
Conversely, methyl formate hydrate can participate in esterification reactions, where it reacts with acids to form esters. This is particularly useful in synthesizing various organic compounds, including fragrances and pharmaceuticals.
Applications of HCOOCH₂H₂O
Industrial Manufacturing
In the industrial sector, methyl formate hydrate is a solvent and intermediate in synthesizing various chemicals. It is used in the production of adhesives, coatings, and plastics.
Environmental Chemistry
Methyl formate hydrate is utilized in environmental chemistry for the degradation of pollutants. Its ability to hydrolyze and break down into simpler compounds makes it effective in treating wastewater and reducing ecological contaminants.
Pharmaceutical Industry
In pharmaceuticals, methyl formate hydrate synthesizes active pharmaceutical ingredients (APIs). Its reactivity allows for the formation of complex molecules necessary for drug development.
Agricultural Sector
Methyl formate hydrate finds applications in agriculture as a fumigant and pesticide. Its efficacy in controlling pests and pathogens makes it valuable in crop protection.
Safety and Handling of HCOOCH₂H₂O
Toxicity and Hazards
Methyl formate hydrate is flammable and toxic if ingested or inhaled. It can irritate the eyes, skin, and respiratory system.
Safety Precautions
When handling methyl formate hydrate, it is essential to use appropriate personal protective equipment (PPE), including gloves, goggles, and respiratory protection. Work should be conducted in well-ventilated areas, and ignition sources should be avoided.
Storage Guidelines
Methyl formate hydrate should be stored in tightly sealed containers, away from heat and direct sunlight. It should be kept in a cool, dry place to prevent decomposition and maintain stability.
Conclusion
HCOOCH₂H₂O, or methyl formate hydrate, is a versatile compound with significant implications and applications across various industries. Its unique chemical structure allows it to participate in various reactions, making it invaluable in chemical synthesis, environmental management, and industrial manufacturing. Understanding its properties, reactions, and applications is crucial for harnessing its full potential in scientific and industrial endeavors.